In the first blog in this series, we discussed the value of dissociated tumor and tissue cells (DTCs) and the important role they play in the development of next-generation immuno-oncology therapeutics including immune checkpoint therapies, targeted immuno-oncology treatments, and biomarker specific immunotherapies. As highly-characterized, primary human biospecimens, DTCs can provide insights into the differences between disease and normal tissues, revealing potential pathways and mechanisms that can be exploited for therapeutic use.
Solid tissue specimens such as tumor samples are not like blood and bone marrowsamples which can be drawn multiple times from the same patient or donor. Tumor resects, where a tumor is surgically removed from a patient’s body typically happens only once, making these diseased samples extremely valuable. As we saw in the first blog of this series, the key to exploiting this value lies in overcoming the time constraints imposed by living tissue samples. These live tissue samples remain viable and useful for experimentation only for a matter of days, forcing researchers to run experiments as samples become available, which is inefficient and wasteful. The rush to start experiments also means that there is insufficient time to properly characterize samples prior to experimentation. There are also the logistical barriers of transporting, processing, and annotating live tissue samples to overcome. In this, the second blog in our series on DTCs we review the best practices for the collection, processing, and analysis of solid tissue and tumor samples, and how to maximize the biological information that can be extracted.
DTCs represent a solution to the time constraint problem. By processing live tissue samples into cryopreserved aliquots of DTCs we can effectively stop the clock. The cryopreserved DTCs remain stable for years in the vapor phase of liquid nitrogen, allowing for extensive phenotypic and genotypic characterization of the DTC samples. Researchers can then select from a population of highly characterized DTC samples and perform experiments in a timeframe determined by the research goals, not by the samples. As we will see in this blog however, the process of collecting samples and processing then into DTCs is challenging, and only best in class execution can guarantee high quality, high viability DTCs suitable for research use.
Processing DTCs on a global scale
To facilitate the processing of DTCs on a global scale Discovery Life Sciences maintains two processing centers, one in the United States and another in Europe, that receive and process tissue specimens from an extensive network of clinical collection sites. This infrastructure allows Discovery to cover two key geographic regions, the US and the European Community, both of which have high population densities and high per capita rates of resection surgeries. Being physically located in each region allows Discovery to receive samples within 24 – 72 hours post-operation. Samples are immersed in tissue transport solution and shipped in controlled temperature shipping containers at 4°C to maintain viability and are processed immediately upon arrival. To date, Discovery has successfully dissociated and processed over 4,500 unique tumors across numerous indications.
An extensive clinical network transfers samples to one of two central processing facilities

The best analytical data comes from best practice sample prep
Processing tissue samples into high-quality, viable DTCs is a non-trivial matter. Tissue samples are delicate, complex mixtures of cells and non-cellular material that must be teased apart and separated to leave only live, viable cells without any background contamination. The quality of this processing is directly linked to the quality of the analytical data that can be derived from a DTC in later experiments.
Upon receipt at our processing centers, samples undergo physical evaluation prior to mechanical reduction (mincing) of the tissue. This homogenized tissue is then passed through a multi-step, proprietary enzymatic and mechanical dissociation process optimized for each different tumor and tissue type. Dissociated samples are then washed and total viable cell populations counted.
To make these crude dissociated cell mixtures amenable to further analysis they must be further processed. Aggregates that will clog flow cytometry columns must be removed and unwanted cell types including myeloid cells present in the tumor microenvironment must be neutralized with a blocking agent to prevent their non-specific binding of analytical antibodies. Red blood cells (RBC) are not lysed by the preparation process for DTCs and must be tagged with an RBC-specific marker such as CD235a as an aid to data interpretation. Finally, to discriminate between live cells and the present dead cells and non-cellular debris generated during dissociation a live/dead cell discriminator should be used. Taken together these steps can dramatically reduce any background, providing clear, readable data on just those live, tumor-specific cells that we want to interrogate.
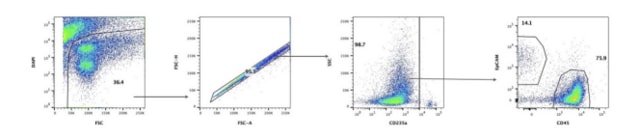

Best practice for measurement and annotation
Aside from the logistical problems that DTCs solve, DTCs also make solid tissue samples amenable to analysis by flow cytometry, a powerful tool for the rapid detection and measurement of cell surface ligands including therapeutically relevant receptors. The process of dissociating primary human tissue generates viable, single-cell suspensions that are amenable to flow cytometry analysis; however, careful handling of these specimens is important to ensure the highest quality data are obtained. In total Discovery Life Sciences has profiled over 4,500 unique tumors by flow cytometry, and best practices for analyzing DTCs have been established to streamline processing and analysis. For these evaluations, tumor cells and total immune cells are profiled, and the immune cell compartment is further evaluated for the presence of CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytic cells, and granulocytic cells.
Through profiling such a large volume of unique dissociated tumors, indication-specific trends are observed, specifically in terms of tumor and immune content, the presence or absence of different cellular subsets, as well as the specific immune cell subsets present in different types of cancer. This information provides important insights for researchers working on the next generation of cancer therapeutics. In our next blog in this series, we will look at some of the tumor-specific expression and population patterns identified by this large-scale immunophenotyping endeavor covering over 4,500 unique tumors.
This blog series is based on the Discovery DTCs User Guide, a comprehensive guide to best practice in DTCs, and details of the indication-specific patterns uncovered by Discovery Life Sciences as part of their work on disease tissue samples. You can download the full pdf from the Discovery Life Sciences website.
References
- Discovery DTCs User Guide, a comprehensive guide to best practice in DTCs. 2022. Discovery Life Sciences. [online] Available at https://www.dls.com/resources/dtcs-user-guide/ [Accessed 15 April 2022]


